Advancing Healthcare Through Innovative Medical Device Software Development
The Importance of Medical Device Software Development
Medical device software development plays a crucial role in the healthcare industry, revolutionizing the way medical devices function and interact with patients and healthcare professionals. As technology continues to advance, the need for sophisticated and reliable software in medical devices becomes increasingly evident.
Enhancing Patient Care
Medical device software development enables the creation of innovative devices that can monitor patient health in real-time, provide accurate diagnostics, and even deliver personalized treatment plans. These advancements not only improve patient care but also contribute to better health outcomes and increased patient satisfaction.
Ensuring Regulatory Compliance
Developing software for medical devices requires strict adherence to regulatory standards and guidelines set forth by organizations such as the FDA. Compliance with these regulations is essential to ensure the safety and effectiveness of medical devices, as well as to protect patient data and privacy.
Addressing Security Concerns
Security is a top priority in medical device software development, given the sensitive nature of patient data and the potential risks associated with cyber threats. Developers must implement robust security measures to safeguard against unauthorized access, data breaches, and other security vulnerabilities.
Embracing Innovation
The field of medical device software development is constantly evolving, driven by technological advancements and innovative solutions. Developers are continuously exploring new technologies such as artificial intelligence, machine learning, and Internet of Things (IoT) to create smarter, more efficient medical devices that can improve patient outcomes.
Collaboration Across Disciplines
Successful medical device software development requires collaboration among multidisciplinary teams comprising software engineers, healthcare professionals, regulatory experts, and quality assurance specialists. By working together seamlessly, these teams can ensure that medical devices meet industry standards while addressing the unique needs of patients and healthcare providers.
Conclusion
In conclusion, medical device software development plays a vital role in shaping the future of healthcare by driving innovation, enhancing patient care, ensuring regulatory compliance, addressing security concerns, and fostering collaboration across disciplines. As technology continues to advance, the importance of developing high-quality software for medical devices will only grow stronger in improving healthcare delivery worldwide.
Understanding Medical Device Software Development: Key Questions and Insights
- What are the 4 phases of medical device development?
- What programming language is used in medical devices?
- Is MDR a software?
- What is software in a medical device?
- What is an example of software in a medical device?
- What is medical software development?
What are the 4 phases of medical device development?
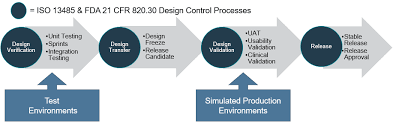
In medical device development, the process typically consists of four key phases: concept and feasibility, design and development, verification and validation, and manufacturing and commercialization. During the concept and feasibility phase, the initial idea for the medical device is explored to determine its viability and potential market impact. The design and development phase involve creating detailed specifications and prototypes based on the concept. Verification and validation ensure that the device meets regulatory requirements, functions as intended, and is safe for use. Finally, in the manufacturing and commercialization phase, the device is produced at scale and brought to market to benefit patients and healthcare providers. Each phase is critical in ensuring the successful development of a safe, effective, and compliant medical device.
What programming language is used in medical devices?
In the realm of medical device software development, various programming languages are utilized to create the sophisticated and reliable software that powers these devices. Commonly used programming languages in medical devices include C, C++, Java, Python, and others. The choice of programming language depends on factors such as the device’s hardware requirements, real-time processing needs, security considerations, and the specific functionalities required for the medical device to operate effectively and safely. Developers carefully select the most appropriate programming language to ensure that the software meets regulatory standards, performs optimally, and delivers accurate results for healthcare professionals and patients.
Is MDR a software?
In the context of medical device software development, it is important to clarify that MDR (Medical Device Regulation) is not a software itself, but rather a regulatory framework established by the European Union to ensure the safety, quality, and performance of medical devices. MDR outlines requirements that medical device manufacturers must adhere to when developing and bringing their products to market. Compliance with MDR is essential for ensuring that medical devices meet the necessary standards for patient safety and effectiveness. Understanding and following the guidelines set forth by MDR is crucial for developers involved in medical device software development to navigate regulatory requirements successfully.
What is software in a medical device?
Software in a medical device refers to the programs or applications that are embedded within the device to control its functions, collect and process data, and provide feedback to healthcare professionals or patients. This software is essential for the operation of modern medical devices, enabling them to perform complex tasks such as monitoring vital signs, delivering treatments, and analyzing patient data. The software in a medical device must meet stringent regulatory requirements to ensure its safety, efficacy, and reliability in clinical settings. It plays a critical role in enhancing patient care, improving diagnostic accuracy, and advancing medical technology to better serve the needs of healthcare providers and patients alike.
What is an example of software in a medical device?
An example of software in a medical device is the software used in a patient monitoring system. This type of software is designed to collect, analyze, and display real-time data from various medical sensors attached to a patient. It provides healthcare professionals with vital information about the patient’s health status, such as heart rate, blood pressure, oxygen saturation levels, and more. The software plays a critical role in helping healthcare providers make informed decisions quickly and accurately, ultimately improving patient care and outcomes.
What is medical software development?
Medical software development refers to the process of designing, creating, and implementing software solutions specifically tailored for use in medical devices and healthcare applications. This specialized field involves developing software that enables medical devices to perform various functions, such as monitoring patient health, analyzing medical data, providing diagnostics, and supporting treatment plans. Medical software development plays a critical role in advancing healthcare technology, improving patient care, ensuring regulatory compliance, and addressing security concerns related to the use of software in medical settings. It requires expertise in both software engineering principles and healthcare industry regulations to create innovative and reliable solutions that meet the unique needs of patients and healthcare professionals.